经历了这么多场考试,宝宝们一定身心俱疲了吧,心疼~送上的爱的抱抱!
胜利的曙光就在前方了,稍作休息之后一定要继续奋斗哦!这次给大家带来2017年化学CIE选择题真题解析,大家要定下心来看完解析哦!
Section A
For each question there are four possible answers, A, B, C and D. Choose the one you consider to be correct. Use of the Data Booklet may be appropriate for some questions.
1. In which species are the numbers of protons, neutrons and electrons all different?

解析:简单题 protons 11,neutrons 12,electrons 10,选项B正确
2. Which would contain 9.03 × 1023 oxygen atoms?
A. 0.25 mol aluminium oxide
B. 0.75 mol sulfur dioxide
C.1.5 mol sulfur trioxide
D.3.0 mol water
解析:简单题 9.03/6.02=1.5mol得到oxygen atoms微粒数,1.5/2=0.75mol 是oxygen molecules的数目,所以B选项正确。
3. In some fireworks there is a reaction between powdered aluminium and powdered barium nitrate. Heat is evolved, an unreactive gas is produced, and all nitrogen atoms are reduced. What is the equation for this reaction?
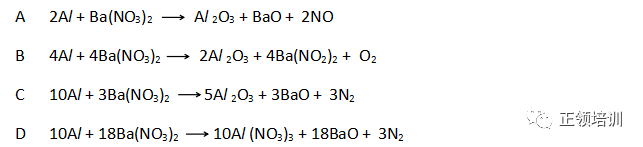
解析:
(1)Barium nitrate is a colorless or white crystal. It is a strong oxidizing agent, a combustion supporting and toxic. It can cause combustion or explosion by contacting with sulfur, phosphorus and organic matter. The melting point is 592 ℃.A green flame is present when burning. Used as an oxidizing agent and an analytical reagent.
(2)活泼性排在Hg以后的金属nitrate分解成metal、NO2、O2,group 1的金属nitrate分解成nitrite和O2 。Group 2的nitrate 比如Barium nitrate分解成NO2、O2和对应的氧化物,NO2、O2与Al发生反应redox reaction,C选项中所有NO3-中的N被Al reduced to N2 ,Al被oxidized生成Al 2O3

4. Which organic compound has the highest boiling point?

解析:
1)Branched chain has lower boiling point,分子越规则,分子间接触面积越大,分子间力越强
2)group的位置,group如果能使整个分子体积增大,分子间距会变大,那么boiling point会降低
3)-OH有hydrogen bond,boiling point会升高(C=O分子间没有形成hydrogen bond)
4)polar molecule会多-dipole forces,boiling point会升高
5)relative molecule mass越大,boiling point越高
6)BCD比A规则,C选项中的C=O会和水形成hydrogen bond,但是boiling point考虑的是分子间力,所以D选项符合题意
5. At a temperature of 2500 K and a pressure of 1.00 ×10–4Pa a sample of 0.321 g of sulfur vapour has a volume of 2.08 × 106m3. What is the molecular formula of sulfur under these conditions?
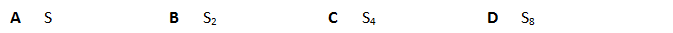
解析:启用PV=nRT 公式, 代入已知条件1.00×10–4×2.08×106=n×8.314×2500K
n(Sx)=0.01mol
n(Sx)=0.321/32=0.01mol
x=1, A选项符合题意
6. Which reaction involves a decrease in the bond angle at a carbon atom?
A. bromoethane refluxed with ethanolic sodium hydroxide
B. complete combustion of methane in air
C. ethanol heated with conc.H2SO4
D. Polymerisation of ethene
解析:A选项 bromoethane(109.5°)生成ethene的bond angle是120°,B 选项 methane(109.5°)生成CO2(180°),C选项 ethanol(109.5°)生成ethene(120°), D选项 ethene(120°)生成polyethylene(109.5°),D选项符合题意。
7. In the high temperatures of car engines, nitrogen reacts with oxygen to give nitrogen monoxide.
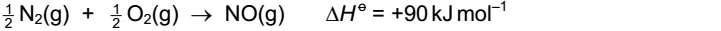
This reaction has activation energy Ea.
Which reaction pathway diagram could correctly represent this reaction?
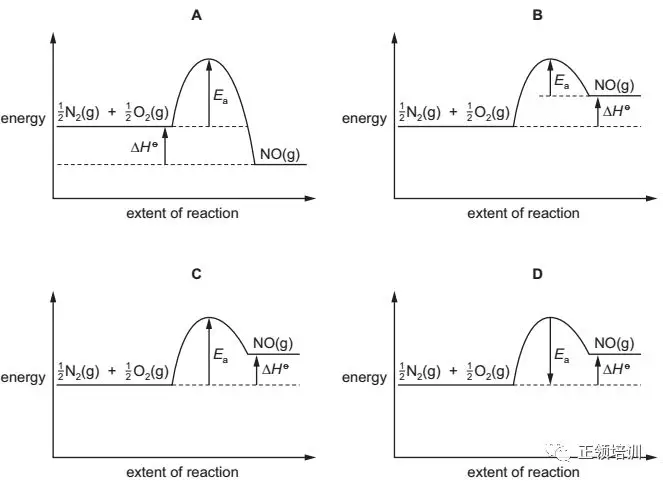
解析:H o = +90 kJ mol–1 >0 可知是endothermic reaction,产物energy高于反应物,A排除。Activation energy :start-up energy‘ pump priming’ the reaction.书上P142,Ea是reactant到最高点的距离,B排除。反应物的能量上升到发生化学反应的活跃状态所需要的能量,这是能量上升的过程,D选项排除。所以C选项符合题意。
8. A reaction sequence is shown.
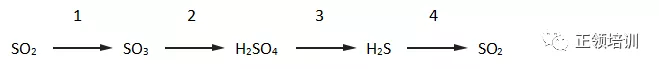
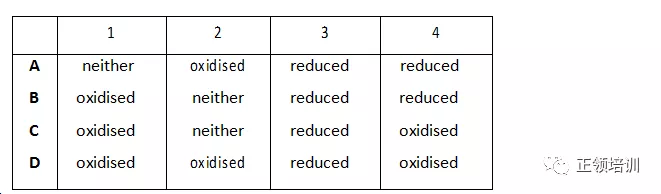
In each stage of this sequence the sulfur is oxidised, reduced or neither oxidised nor reduced. Which row is correct?
解析:

+4到+6(lose electrons-oxidised),+6到+6( neither),+6到-2(gain electrons-reduced ),-2到 +4(lose electrons-oxidised),C选项符合题意。
9. Hydrogen and carbon dioxide gases are mixed at 800 K. A reversible reaction takes place.
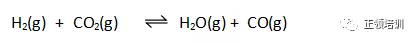
At equilibrium, the partial pressures of H2 and CO2 are both 10.0 kPa. Kp is 0.288 at 800 K. What is the partial pressure of CO in the equilibrium mixture?
A.5.37 kPa B. 18.6 kPa C. 28.8 kPa D. 347 kPa
解析: Kp = 0.288 = x2/10.0×10.0
x = 5.37 ,A选项符合题意
10. A reaction involved in the Contact process is shown.

The reaction is investigated at 200 kPa and 700 K and the value of the equilibrium constant, Kp, is found to be Y. The reaction is then investigated at 1000 kPa and 700 K and the value of Kp is found to be Z. Which statement comparing Y and Z is correct?
A. Y and Z are the same.
B. Y is greater than Z.
C. Z is 2.2 times greater than Y.
D. Z is 5.0 times greater than Y.
解析: 压力从200 kPa 增加到1000 kPa ,温度一直保持是700 K,the chemical equilibrium constant(Kp)is only related to temperature. The equilibrium constant of endothermic reaction increases with the increase of temperature, whereas the exothermic reaction is the opposite.A选项符合题意。
11.The Boltzmann distribution for the hydrogenation of an alkene at a particular temperature in the absence of a catalyst is shown.
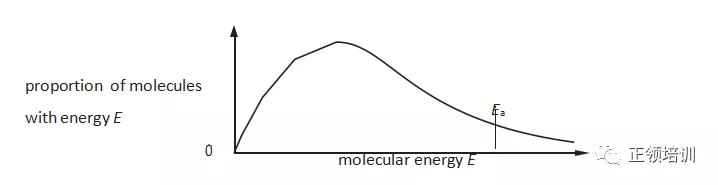
Which row correctly describes the effects of adding nickel to the reaction vessel?
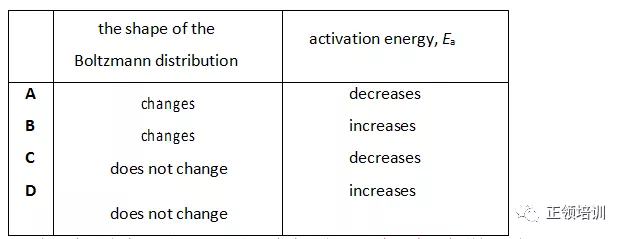
解析:因为温度没有变,所以shape也不会变,如果温度升高Ea会增加。书P142 ,a catalyst does this by making it possible for the particles to react by an alternative mechanism.This alternative mechanism has a lower activation energy (Ea). C选项符合题意
12.The elements magnesium and sulfur each form doubly charged ions. How do the atomic radii and ionic radii of these elements compare?
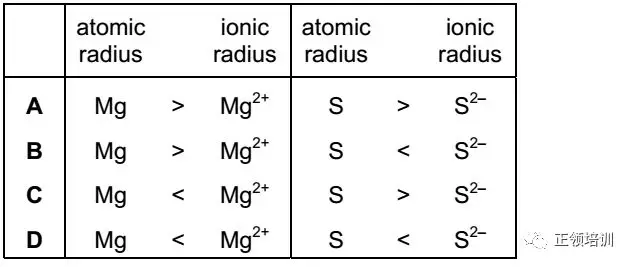
解析:Mg和Mg2+的proton number一样,Mg electronic configuration282,Mg2+ 是28,Mg2+ 少一个电子层,所以radius小。S和S2-的proton number一样,对电子的吸引力就没差别,S的electronic configuration286,S2-是288结构,电子层数相同,但是S2-电子数更多,比S更难束缚,所以radius大。所以,B选项符合题意。
13.Which graph correctly shows relative electronegativity plotted against relative atomic radius for the elements Na, Mg, Al and Si ?
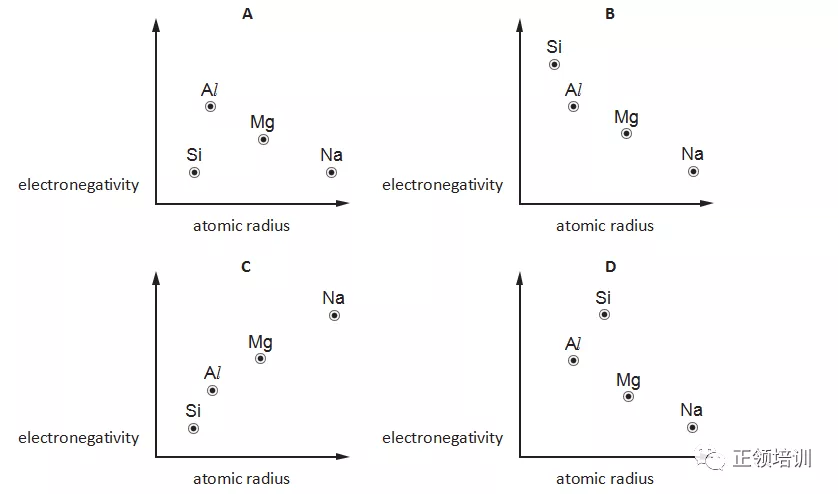
解析:electronegativity肯定是non-metal比较厉害,A和C先排除。在同一period,从左向右atomic radius逐渐减小,B选项符合题意。
14.Trends are seen in the physical and chemical properties of the elements of Group 2 and their compounds. Which property shows a decrease from magnesium to barium?
A. the rate of the reaction between the element and dilute hydrochloric acid
B. the solubility of the hydroxides
C. the solubility of the sulfates
D. the temperature of decomposition of the carbonates
解析:the rate of the reaction between the element and dilute hydrochloric acid应该是增加的,element越往下越活泼,A选项排除。溶解性Be(OH)2 < Mg(OH)2 < Ca(OH)2 < Sr(OH)2 < Ba(OH)2,B也排除。BeSO4 > MgSO4> CaSO4> SrSO4 >BaSO4,C选项符合题意。element越活泼,carbonates越难分解(decompose)。
15.Calcium oxide is added to water and the resulting mixture is filtered. This filtrate is X. When carbon dioxide is bubbled through filtrate X, a white precipitate is formed. Which equation for this reaction of filtrate X with carbon dioxide is correct?
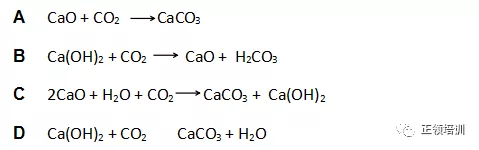
解析:Calcium oxide is added to water
filtrate X 就是Ca(OH)2,D选项符合题意。
16. Element X reacts with cold, dilute, aqueous sodium hydroxide to form two different chlorine-containing products, Y and Z. What are the oxidation states of chlorine in Y and Z?
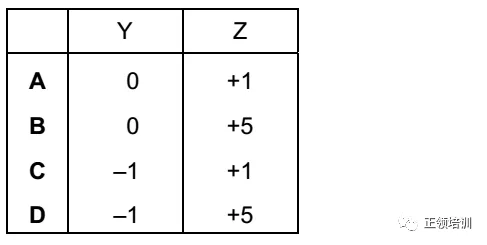
解析:氯气与冷的sodium hydroxide生成Sodium Hypochlorite: Cl2+2NaOH=NaCl+NaClO+H2O,与热的sodium hydroxide生成Sodium chlorate: Cl2+6NaOH=5NaCl+NaClO3+3H2O,C选项符合题意
17. A powder is known to be either a single sodium halide or a mixture of two sodium halides. A sample of the powder was dissolved in water.
Aqueous silver nitrate was added, and a pale yellow precipitate was formed. When concentrated aqueous ammonia was then added, this precipitate partly dissolved leaving a darker yellow precipitate. What might the powder be?
A. sodium bromide only
B. sodium iodide only
C. a mixture of sodium chloride and sodium bromide
D. a mixture of sodium chloride and sodium iodide
解析:pale yellow precipitate是AgI、AgCl,Ag+ + 2NH3·H2O = Ag(NH3)2 + +2H2O
2Ag+ + 2NH3·H2O = Ag2O↓ + 2NH4+ + H2O ,darker yellow precipitate就是Ag2O。D选项符合题意
18.The ammonium ion is formed by the following reaction.
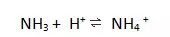
Which statement about the species involved in this reaction is correct?
A The ammonia molecule contains a dative covalent bond.
B The ammonium ion is a Brønsted-Lowry base as it has accepted a proton.
C The H–N–H bond angle changes from 107° in ammonia to 90° in the ammonium ion.
D The number of electrons surrounding each nitrogen atom does not change.
解析:A选项The ammonium ion contains a dative covalent bond and ammonia molecule contains Polar covalent bond.所以A排除。Brønsted-Lowry acid-base theory:when an acid and a base react with each other, the acid forms its conjugate base, and the base forms its conjugate acid by exchange of a proton (the hydrogen cation, or H+)。HA + B ⇌ A− + HB+,Acid:H+ 的doner,base:H+ 的acceptor, ammonium ion不能再接受H+,只能给出H+,是Brønsted-Lowry acid,B排除。The H–N–H bond angle changes from 107° in ammonia to 109°28’ in the ammonium ion,C排除。D选项符合题意
19. A chemist took 2.00 dm3 of nitrogen gas, measured under room conditions, and reacted it with a large volume of hydrogen gas to produce ammonia. Only 15.0% of the nitrogen gas reacted to produce ammonia. Which mass of ammonia was formed?
A. 0.213 g B. 0.425 g C. 1.42 g D. 2.83 g
解析:V(N2)= 2.00×0.15 = 0.3 L(参加反应的N2),根据N2(g)+3H2(g)⇌2NH3(g),V(N2):V(NH3)= 1:2,生成V(NH3)=0.6 L,m=n×M=V(NH3)/Vm ×17 = 0.425 g,B选项符合题意
20.A carbonyl compound X will react with HCN in the presence of NaCN to make a compound with Mr 85. Compound X does not react with Fehling’s reagent. What is X?
A. Butanal B. butanone C. propanal D. propanone
解析:ABCD(C=O、HC=O)都可以和HCN反应,其中只有C选项propanal CH3CH2CHO和D选项CH3COCH3(C=O)和HCN反应生成 Mr =85的compound ,A和B排除,B选项CH3COCH2CH3和D选项CH3COCH3(C=O)都不可以和Fehling’s reagent反应,A和C排除。所以D选项符合题意
21. Geraniol and linalool are compounds found in some flower fragrances.
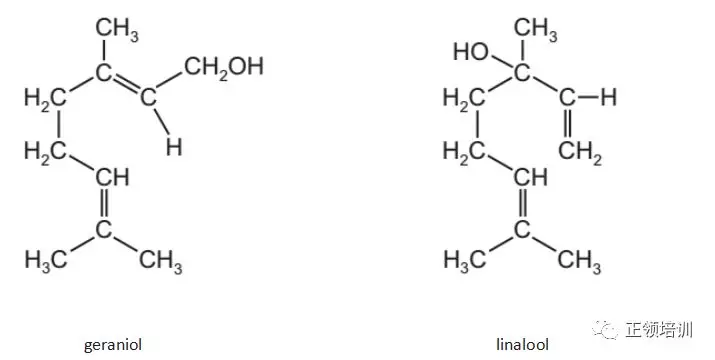
Which statement is correct?
A. They are chain isomers of each other.
B. They are geometrical isomers of each other.
C. They are optical isomers of each other.
D. They are positional isomers of each other.
解析:structural isomerism包括:position isomerism、functional group isomerism、chain isomerism。Stereoisomerism包括:cis-trans isomerism(geometric isomerism)、optical isomerism
1)chain isomers:hydrocarbon chains have variable amounts of branching
2)functional group isomers:different functional groups present
3)optical isomers:the two different molecules are mirror images of each other and cannot be superimposed ,they differ in their effect on polarised light
4)cis-trans isomerism(geometric isomerism):are used to describe any molecules with restricted rotation in the molecule. For molecules with C=C double bonds, these descriptors describe relative stereochemistry only based on group bulkiness or principal carbon chain, and so can be ambiguous.
5)positional isomers :it is the position of the functional group that varies in each isomer。 D选项符合题意。
22.Which equation represents the initiation step of the substitution reaction between methane and chlorine?
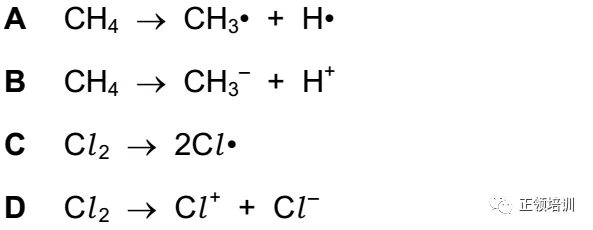
解析:书P206,free radical substitution分三步:第一步(共价键断裂产生自由基是Initiation stage)
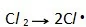
第二步Propagation step:引发阶段(Initiation stage)产生的自由基与反应体系中的分子作用,产生一个新的分子和一个新的自由基,新产生的自由基再与体系中的分子作用又产生一个新的分子和一个新的自由基,如此周而复始、反复进行的反应过程称为链(式)反应。
第三步:two free radicals meet they will react with each other 。C选项符合题意
23. Aqueous sodium hydroxide reacts with 1-bromopropane to give propan-1-ol.
What should be included in a diagram of the first step in the mechanism?
A. a curly arrow from a lone pair on the OH– ion to the Cd+ atom of 1-bromopropane
B. a curly arrow from the Cd+ atom of 1-bromopropane to the OH– ion
C. a curly arrow from the C–Br bond to the C atom
D. the homolytic fission of the C–Br bond
解析:
the heterolytic fission of the C–Br bond,只有A选项符合题意。
24. A sample of 2.76 g of ethanol was mixed with an excess of aqueous acidified potassium dichromate(VI). The reaction mixture was then boiled under reflux for one hour. The required organic product was then collected by distillation.The yield of product was 75.0%. Which mass of product was collected?
A. 1.26 g B. 1.98 g C. 2.07 g D. 2.70 g
解析:M(CH3CH2OH)=46 M(CH3COOH)=60, 2.76g × 75% = 2.07 g
n(CH3CH2OH)= m/M= 2.07g/46 =0.045mol = n(CH3COOH)
m(CH3COOH)= n × M = 0.045 × 60 =2.70 g , D选项符合题意。
25.Compound X is a single, pure, optical isomer. X is heated with an excess of concentrated H2SO4. Only one organic product is formed. What could X be?
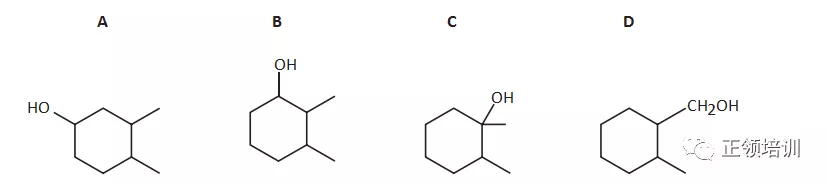
解析:The carbon atom with four different groups attached is called the chiral centre of the molecule. ABC的产物不止一种,只有D符合题意。
26. 2-bromo-2-methylpropane undergoes nucleophilic substitution when heated under reflux with an aqueous solution of sodium hydroxide. Which row is correct?
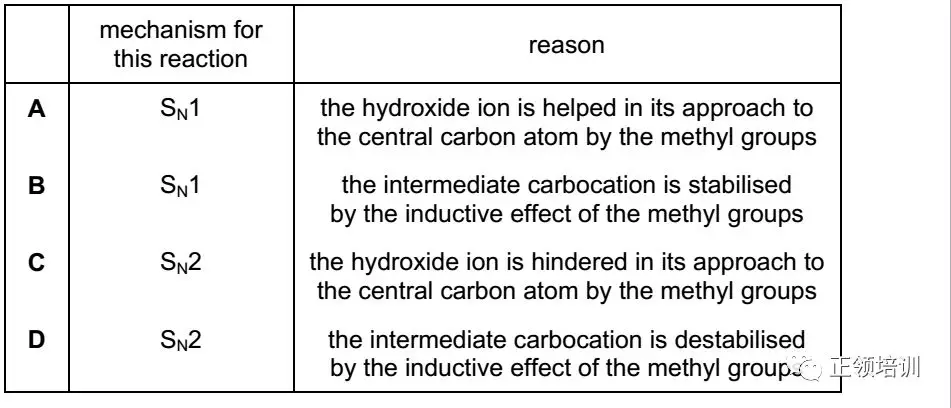
解析:
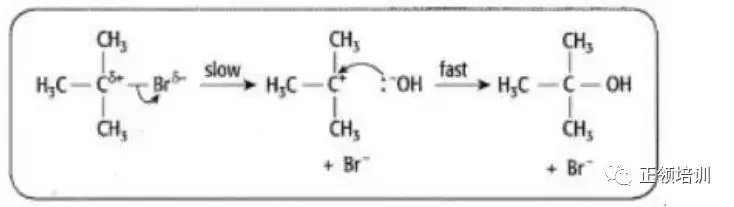
根据2-bromo-2-methylpropane的结构,判断出是SN1 ,A选项描述的reason有误,B选项符合题意。
27. H2NNHC6H3(NO2)2 is the structural formula of 2,4-DNPH.Many, but not all, organic reactions need to be heated before reaction occurs. Which reaction occurs at a good rate at room temperature (20°C)?
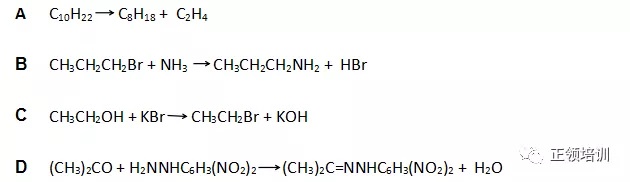
解析:A选项需要高温Cracking is a process in the process of petrochemical production, with a higher temperature than cracking (700 - 800, or even up to 1000 degrees centigrade), making the long chain hydrocarbons in the products of petroleum fractionation (including petroleum gas) break into short chain hydrocarbons such as ethylene and propylene.B选项书上P220,bromoethane 生成ethylamine需要加热。C选项书上P227,the alcohol is heated under reflux with the reactants to make the halogenoalkane。D选项反应自发进行,符合题意。
28. A carboxylic acid, P, has no possible chain isomers. It reacts with an alcohol, Q, that has only one positional isomer.What could be the ester formed from a reaction between P and Q?
A. butyl propanoate
B. ethyl butanoate
C. pentyl ethanoate
D. propyl pentanoate
解析:形成B和D的carboxylic acid都有chain isomers,所以排除。形成C的alcohol有两个positional isomer,形成A的alcohol只有一个positional isomer,所以A符合题意。
29. Which compound is chiral and reacts with Na2CO3 to give CO2?
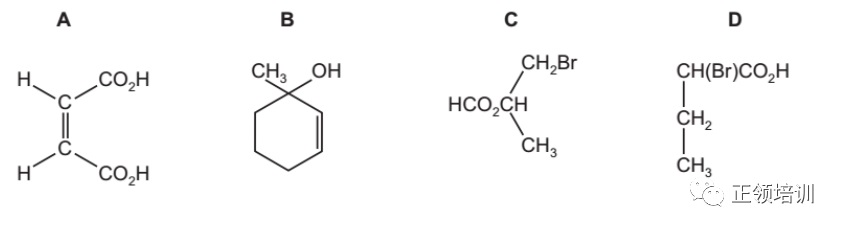
解析:A选项有double bond 不是chiral,B选项是chiral但是酸性不够,不能和
Na2CO3反应。C选项是Ester不是acid,不能和Na2CO3反应生成CO2,只有D选项符合题意。
30. Which compound, when hydrolysed, gives propanoic acid and propan-2-ol?
A. CH3CH2CO2CH2CH(CH3)CH3
B. (CH3)2CHCO2CH2CH3
C. CH3CH2CO2CH(CH3)CH3
D. CH3CH2CH2CO2CH(CH3)CH3
解析:A选项CH3CH2COOH和HOCH2CH(CH3)CH3,B选项(CH3)2CHCOOH和HOCH2CH3, C选项CH3CH2COOH和HOCH(CH3)CH3符合题意,D选项CH3CH2CH2COOH和HOCH(CH3)CH3
Section B
31. An isolated gaseous atom of element X has paired electrons in at least one of its 3d orbitals and has a filled 4s subshell. What could be the identity of element X?
1. iron 2. gallium 3. copper
解析:

32. Which allotropes of carbon have a giant molecular structure?
1. buckminsterfullerene
2. diamond
3. graphite
解析:1是C60属于Molecular crystal,2,3都是giant molecular structure,C选项符合题意
33. Which statements about endothermic reactions are correct?
1. On the reaction pathway diagram the products of the reaction are lower than the reactants.
2. There is a net transfer of heat energy from the surroundings to the reacting system.
3. the total bond energies of the reactants > the total bond energies of the products
解析:1不正确,On the reaction pathway diagram the products of the reaction are higher than the reactants. 2,3都正确,C选项符合题意
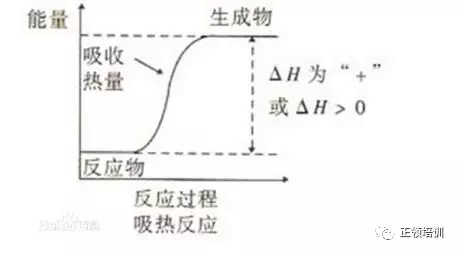
34. The rate of chemical reactions can be increased by the addition of a suitable catalyst. For which reactions can a heterogeneous catalyst be used?
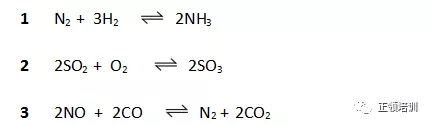
解析:1 的 catalyst是 Fe,2 的 catalyst是V2O5,3中Rhodium and platinum coating as catalyst,所以A选项符合题意。
35. Which chlorides, when added to water, can produce a solution with a pH of less than 5?
1. SiCl/4 2. A/ C/3 3. MgC/2
解析:
1)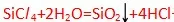
书上P159,the SiO2 is seen as an off-white precipitate. Some of the hydrogen chloride gas produced dissolved in water,leaving an acidic solution(hydrochloric acid,PH<3)显酸性
2)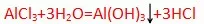
(因为Al(OH)3不在solution中了,hydrochloric acid,PH<3)显酸性
3)MgCl2+2H2O = Mg(OH)2+2HCl发生Incomplete hydrolysis,只能生成少量hydrochloric acid,不一定pH of less than 5。B选项符合题意
36. Acid rain continues to be a problem.Which statements about acid rain are correct?
1. Acid rain is formed when oxides of nitrogen or oxides of sulfur react with water in the atmosphere.
2. Acid rain causes an increase in the concentration of heavy metal ions in water courses.
3. Nitrogen dioxide will catalyse the formation of SO3 from SO2 in the atmosphere.
解析:(1)正确(2)正确Soil acidification increased the solubility of heavy metals, the smaller the PH, the lower the capacity of soil buffer acid rain, and the lower the activity of heavy metals.(3)正确,SO2 + NO2 → SO3 + NO ,A选项符合题意
37. Which compounds contain a chiral centre?
1. 2-hydroxybutanoic acid
2. 3-hydroxybutanoic acid
3. 4-hydroxybutanoic acid
解析:CH3CH2C *HOHCOOH和CH3C*HOHCH2COOH有chiral centre,B选项符合题意
38. The diagram shows the monomer used to make polyvinyl chloride, PVC.
Assuming that one particular molecule of the polymer forms from n molecules of the monomer (where n is many thousands), which statements are correct?
1. The relative molecular mass of this polymer molecule is approximately 62.5n.
2. There are n chiral carbon atoms in this polymer molecule.
3. There are 5n σ bonds in one polymer molecule.
解析:1. 正确,2 正确,3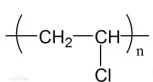
第一个C算1+1+0.5+0.5=3个bond,第二个C算1+1+0.5+0.5=3个bond,应该是6n σ bonds,所以B选项符合题意
39. An organic compound, X, has the following skeletal formula.
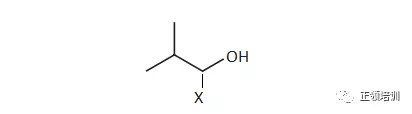
Which statements about X are correct?
1. X is a primary alcohol.
2. X will dehydrate to give a single alkene.
3. X will undergo a substitution reaction with chloride ions.
解析:1正确,2正确(elimination reaction condition:ethanol+ NaOH) 3.可以的,书上P227,CH3CH2OH+HCl----催化剂--->CH3CH2Cl+H2O,Reflux heating in the presence of anhydrous zinc chloride / aluminum chloride。书上P219,CH3CH2Cl+H2O----NaOH--->CH3CH2OH+HCl (condition:aqueous NaOH),A选项符合题意
40. 2,2,4-trimethylpentanal is used in the manufacture of adhesives. Which reagents would 2,2,4-trimethylpentanal react with?
1. 2,4-dinitrophenylhydrazine reagent
2. Tollens' reagent
3. alkaline aqueous iodine
解析:

超越化学反应结果的每一位CIE考生都是值得珍惜的奇迹,祝愿同学们都能成为最好的自己!




